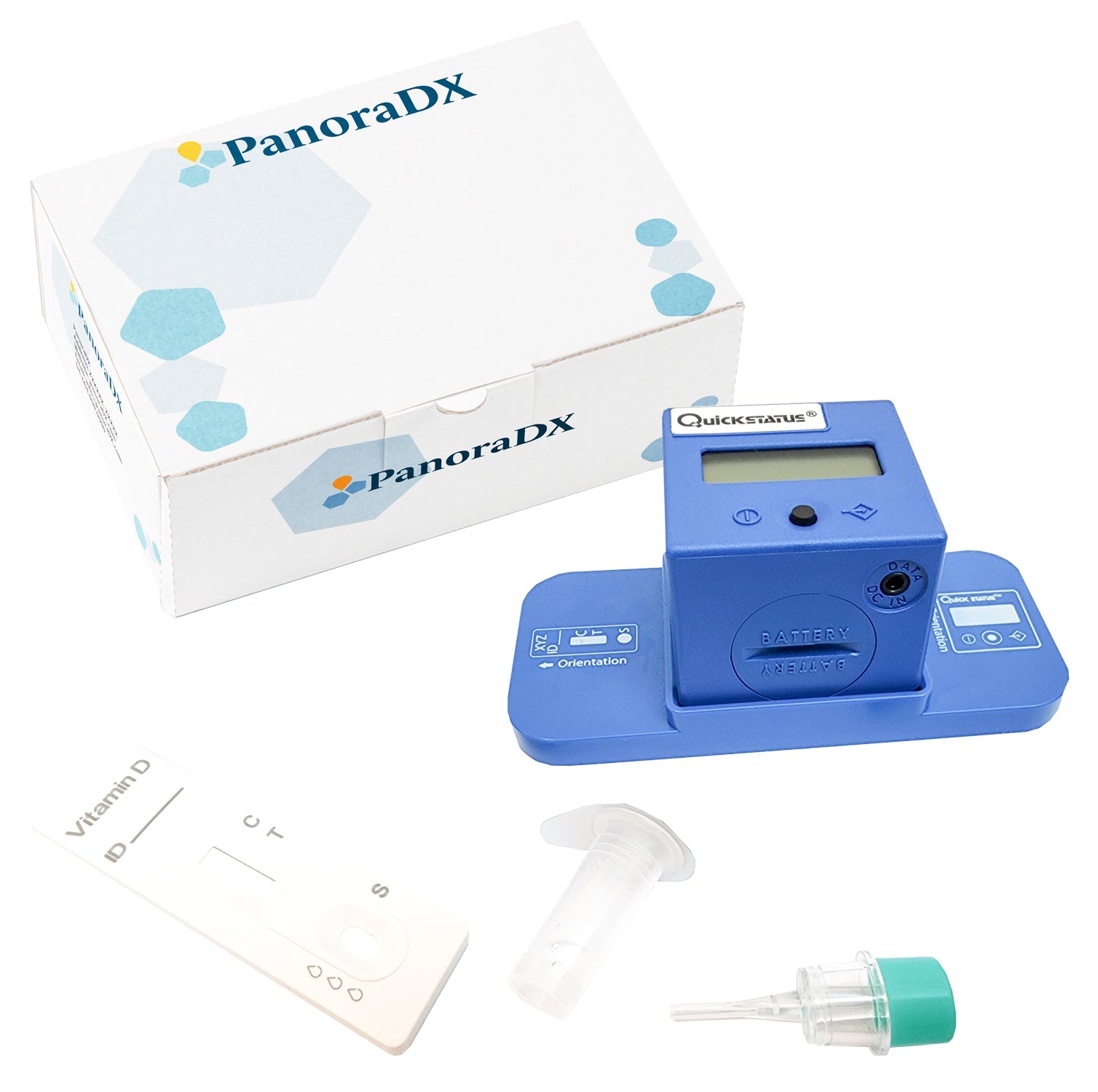



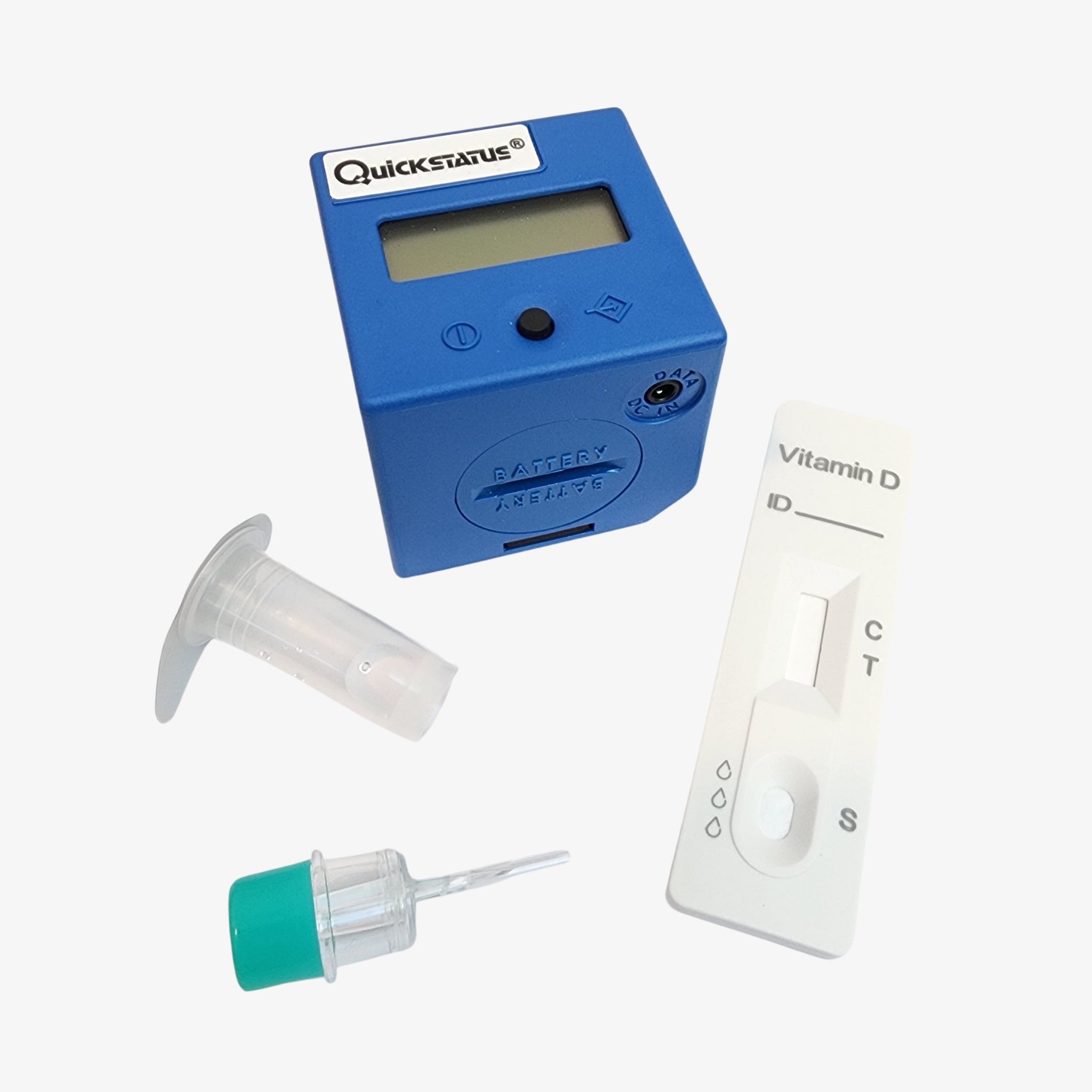







PANORADX STARTER BUNDLE
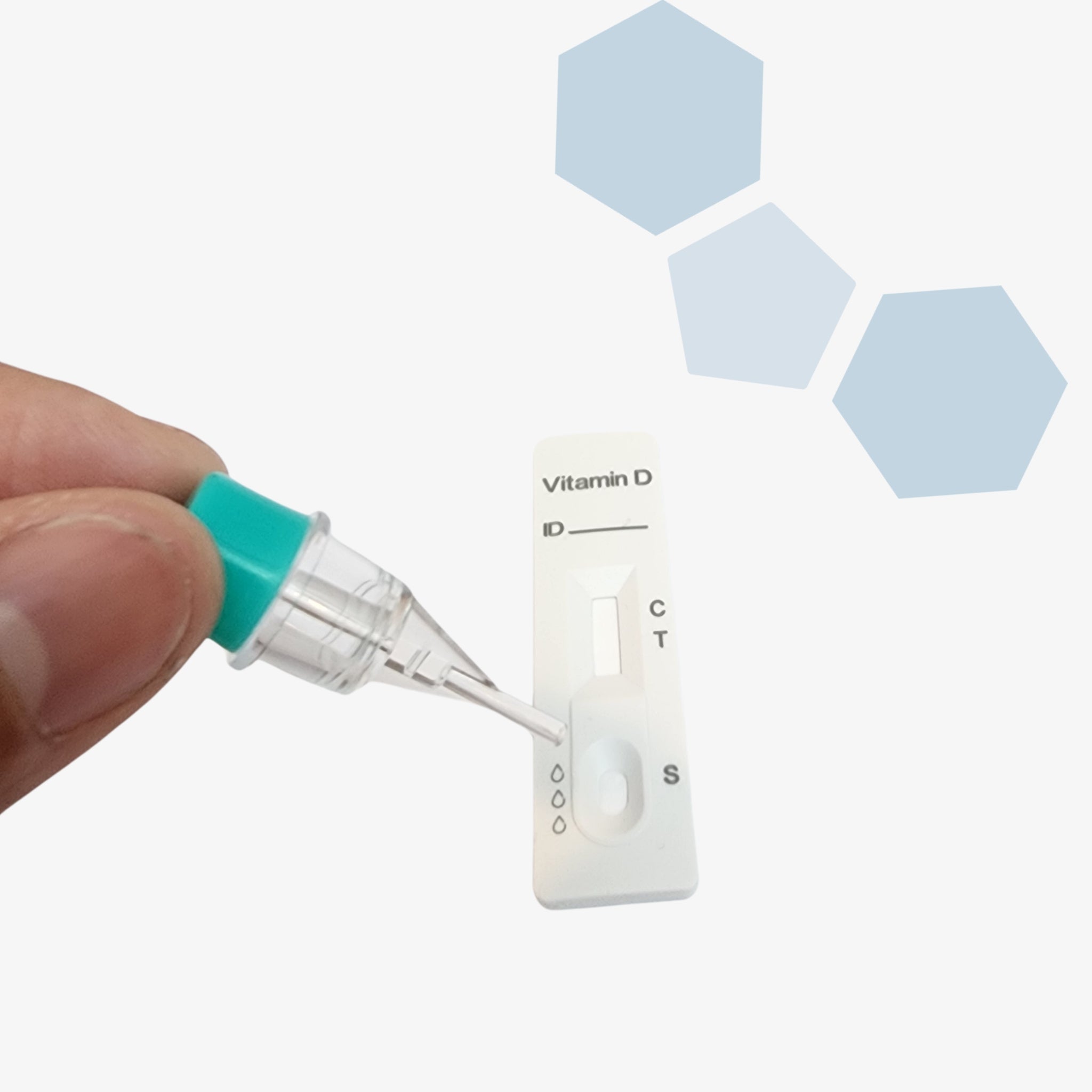
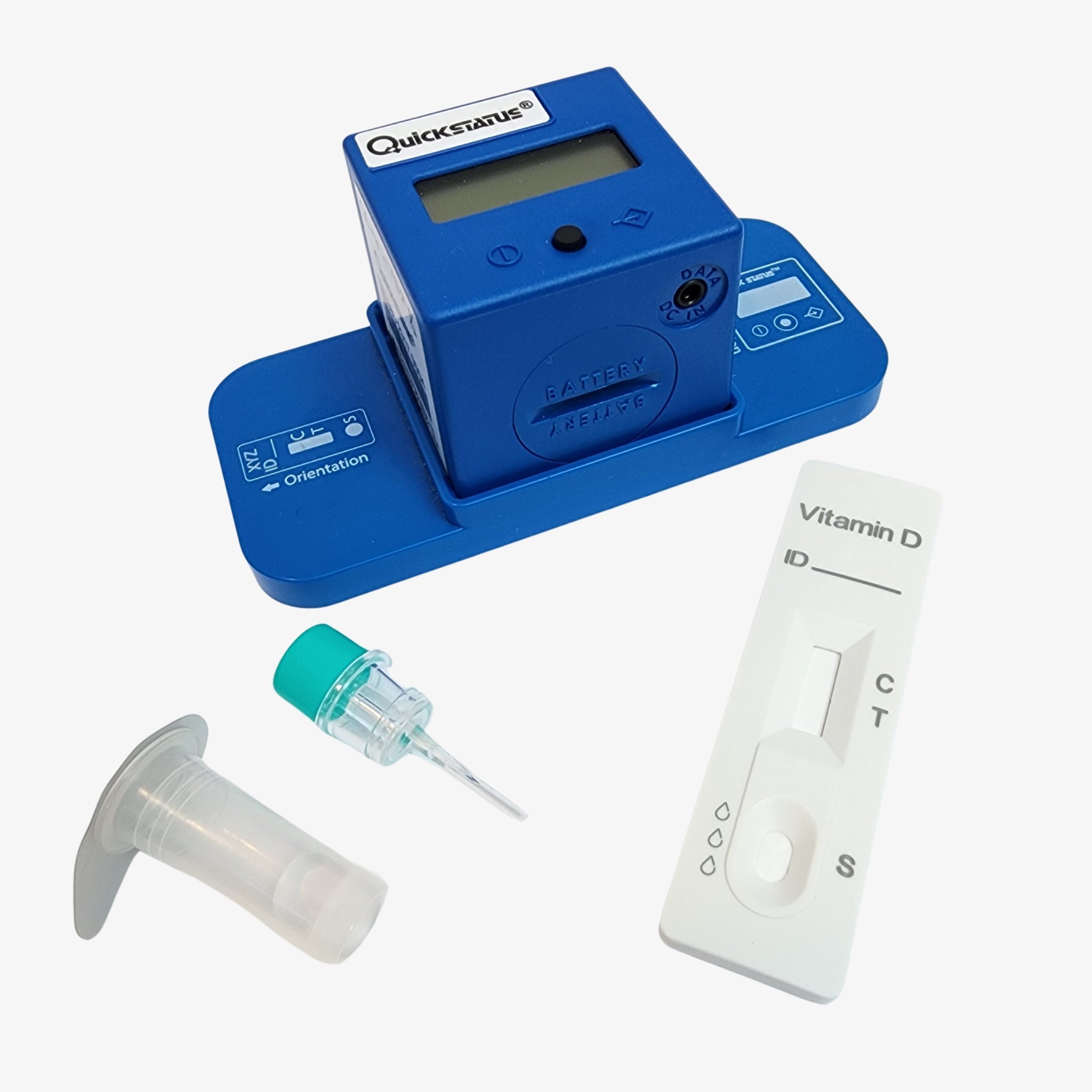
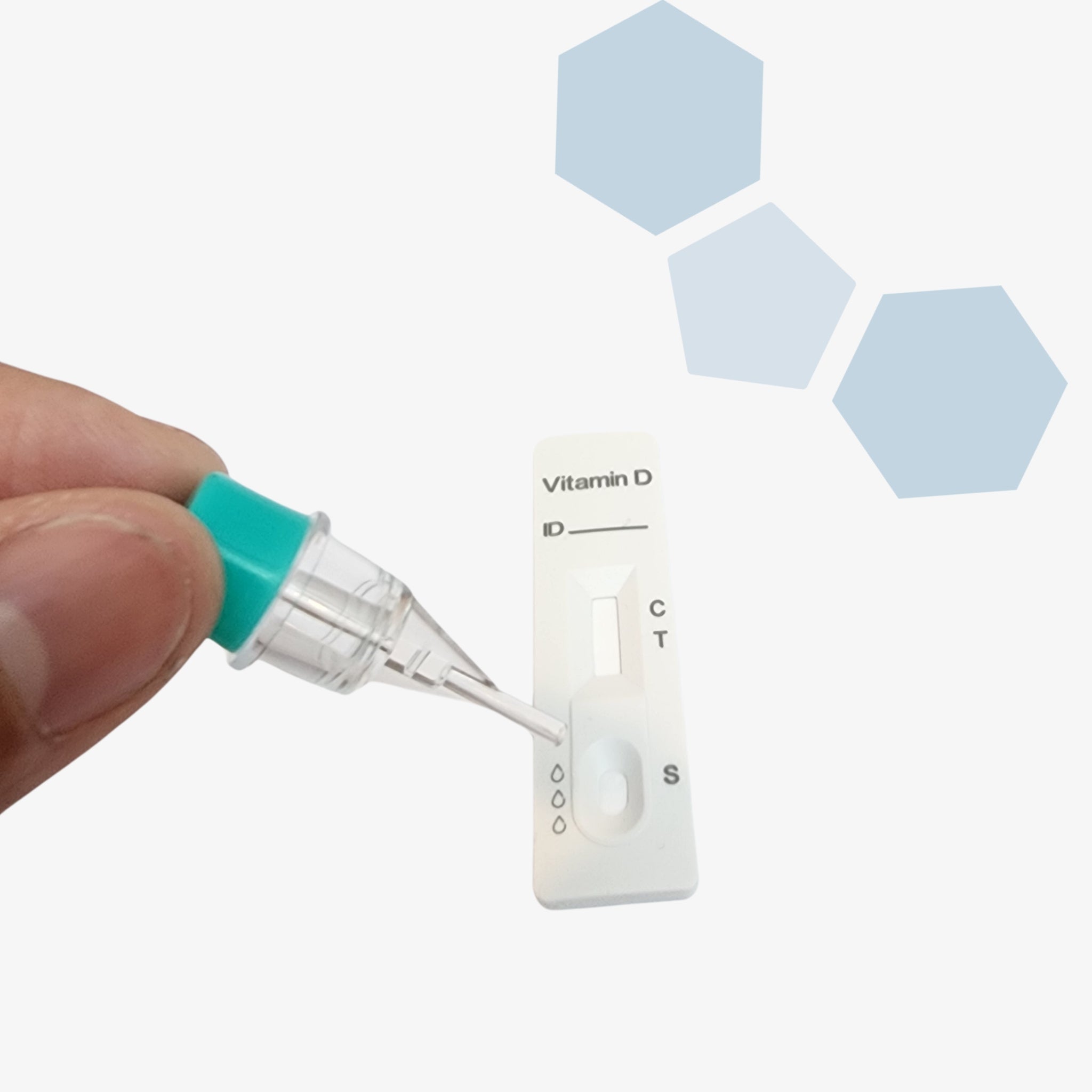
Ensure your dental implant outcomes with the PanoraDX Starter Bundle — a comprehensive, point-of-care solution for rapid, quantitative Vitamin D testing, including 25 tests and a Cube Reader. Designed for dental professionals, this kit provides everything you need to obtain accurate 25-hydroxy Vitamin D levels quickly, empowering you to make...
Special Offer
- PDC Special $200 Off Starter Bundle - Use Code: PDC2025 during checkout Shop the Starter Bundle